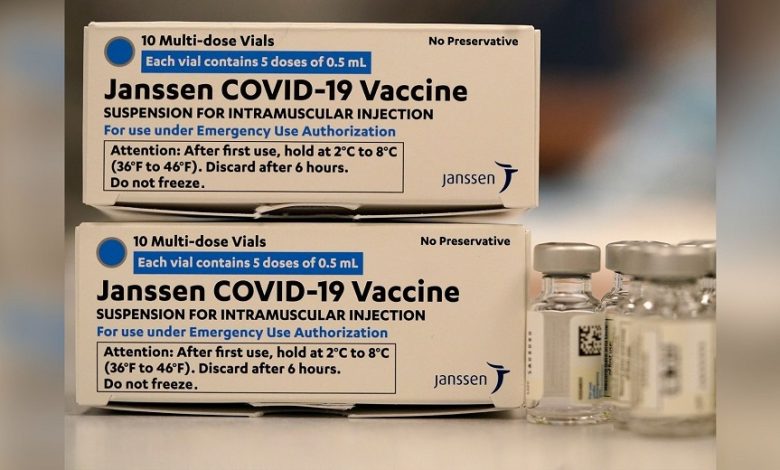
Because of a rare but dangerous risk of blood clots associated with Johnson & Johnson’s Covid-19 vaccine, U.S. authorities on Thursday strictly restricted who might get the vaccine.
According to the Food and Drug Administration, the vaccine should only be administered to people who are unable to obtain a different vaccine or who voluntarily select J&J’s vaccine as an option. The Pfizer or Moderna vaccines should be used instead of the J&J vaccine, according to United States officials, who have been recommending this for months.
J&J’s vaccine will now be limited by the FDA because of new information about the risk of life-threatening blood clots two weeks after vaccination, the FDA said in a statement.
The decision is the latest in a string of restrictions placed on Johnson & Johnson’s one-dose vaccination, which has long been dominated by the more efficient two-shot vaccines from Pfizer and Moderna.
Because of safety concerns, the Centers for Disease Control and Prevention (CDC) suggested that the Moderna and Pfizer vaccines be given priority over the J&J vaccine in December. Previously, authorities in the United States regarded all three vaccinations in the same way since they had all been proven to provide significant protection.
However, follow-up tests have repeatedly shown that J&J’s vaccine is less effective. In addition, although the blood clots associated with J&J’s shot are uncommon, experts believe they are nonetheless happening.




Leave a Reply